Insights+: The US FDA New Drug Approvals in July 2022
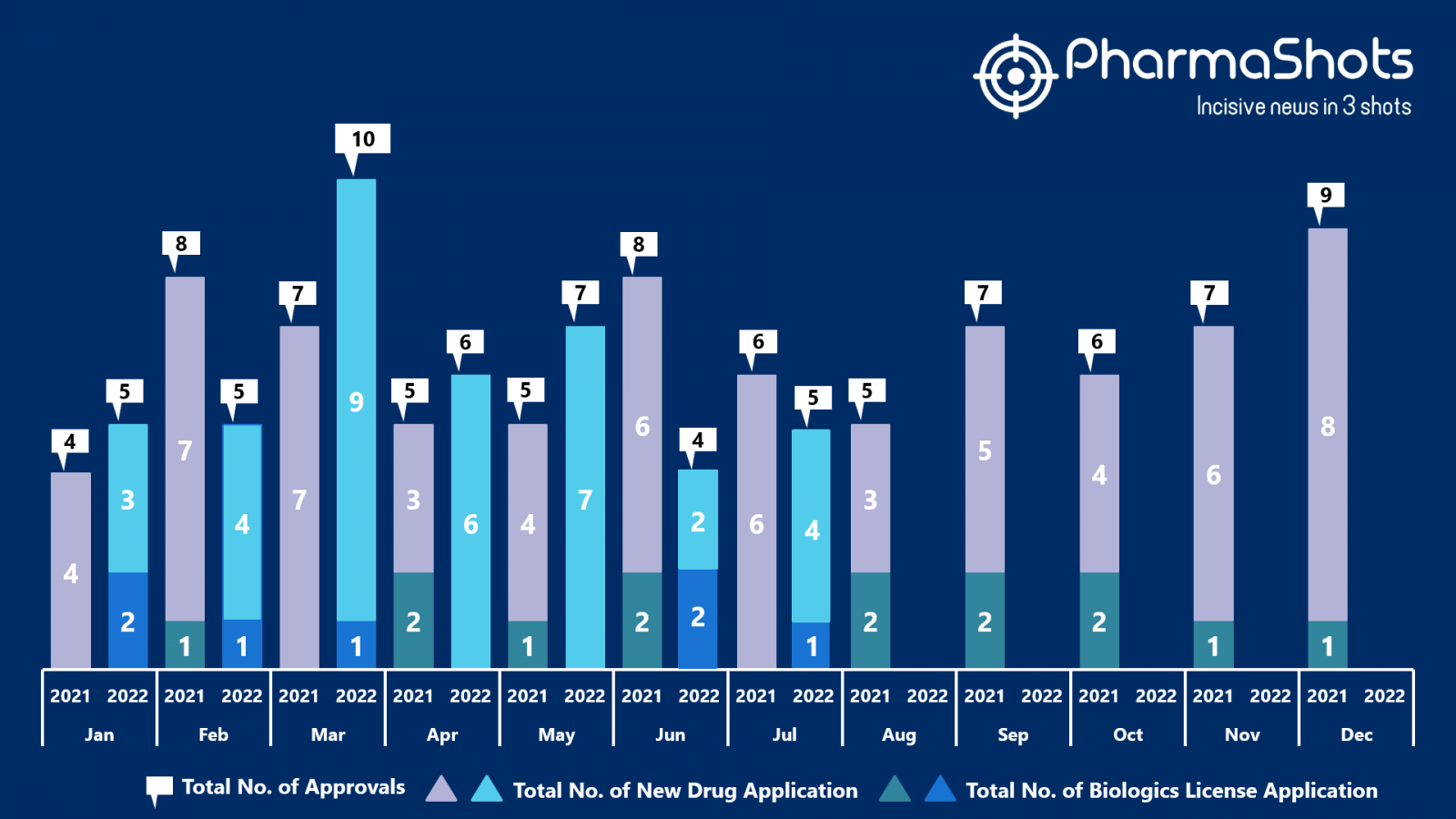
- The US FDA approved 4 NDAs and 1 BLA in July 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 42 novel products in 2022
- In July 2022, the major highlights drugs were Zonisade’s approval for partial seizures in adults and pediatric patients with epilepsy, Opzelura (ruxolitinib) for Vitiligo, Kyzatrex for Hypogonadism
- PharmaShots has compiled a list of a total of 5 new drugs approved by the US FDA in July 2022
Zoryve
Active ingredient: roflumilast Approved: July 1, 2022
Company: Arcutis Biotherapeutics Disease: Plaque Psoriasis
- The US FDA has approved the NDA for Zoryve (0.3%) for PsO, incl. intertriginous areas in patients aged ≥12yrs. The therapy is expected to be available in mid-August
- The therapy has been shown to rapidly clear plaques & reduce itch across all areas of the body & was found to be safe & well tolerated in multiple clinical trials with improvements in disease clearance in hard-to-treat areas
- The company has launched a ZORYVE patient support program that provides access to commercially insured patients to use Zoryve & offers the payer process, lowering the out-of-pocket cost for eligible patients. The company offers Arcutis Cares PAP which will give Zoryve to financially qualified patients who are uninsured or underinsured at free cost
Zonisade
Active ingredient: zonisamide Approved: July 18, 2022
Company: Azurity Pharmaceuticals Disease: Epilepsy
- The US FDA has approved Zonisade (100mg/5mL) for partial seizures in adults & pediatric patients aged ≥16yrs. with epilepsy who have difficulty in swallowing or are unable to take tablets
- In the three trials, the efficacy & tolerability of zonisamide has been evaluated. The first study showed significant treatment differences across 100/200/400mg dose levels while in 2nd & 3rd studies, significant differences across 400mg to 600mg dose with no apparent difference b/w qd & BID
- In an additional analysis of the first 4wks. of treatment, significant differences from PBO b/w 100 & 400mg dose. Zonisade marks the first US FDA-approved oral liquid formulation of zonisamide that helps to reduce the burden & improve treatment adherence
Incyte’s Opzelura (ruxolitinib) Receives the US FDA’s Approval for the Treatment of Vitiligo
Opzelura
Active ingredient: ruxolitinib Approved: July 19, 2022
Company: Incyte Disease: Vitiligo
- The approval was based on the P-III (TRuE-V1 & V2) trial evaluating the safety and efficacy of Opzelura vs vehicle in 600+ patients aged ≥12yrs. with nonsegmental vitiligo
- The results showed a significant improvement in VASI scores representing improvements in facial and total body repigmentation @24 & 52wk. The results were consistent across both studies @24wk., ~30% vs 8% and 13% in (TRuE-V1 & V2) trial & ≥15% vs 2% of patients achieved ≥75% & ≥90% improvement from baseline in F-VASI75 & F-VASI90 @24wk.; ~50% & ~30% @52wk.
- The company also launched a patient support program i.e., IncyteCARES that provide access to eligible patients to use Opzelura & also offers financial assistance, ongoing education, and resources
Benlysta
Active ingredient: belimumab Approved: July 27, 2022
Company: GSK Disease: Active Lupus Nephritis
- The US FDA has approved Benlysta (BLyS-specific inhibitor) for the treatment of active LN who received standard therapy in children aged 5 to 17yrs.
- The main objective of treating lupus nephritis in both adults and children is to maintain renal function while reducing side effects from treatment and associated morbidity
- Benlysta is a human mAb that binds to soluble BLyS. The therapy has the potential to suppress B cell survival incl. autoreactive B cells and slows down the development of B cells into immunoglobulin-producing plasma cells by binding to BLyS
Kyzatrex
Active ingredient: testosterone undecanoate Approved: July 31, 2022
Company: Marius Pharmaceuticals Disease: Hypogonadism
- The US FDA has approved Kyzatrex, an oral testosterone replacement therapy in adult males with hypogonadism. The product is supplied as 100/150/200mg
- The approval was based on the P-III (MRS-TU-2019EXT) study that evaluated Kyzatrex in 155 hypogonadal males aged b/w 18 & 65yrs. which showed that 88% of patients achieved a 24hr. mean plasma total testosterone concentration within the normal range of 222-800ng/dL on the final PK visit @90 day & had a maximum total testosterone concentration threshold ≤1200ng/dL b/w 1400 & 2000ng/dL, & ≥2000ng/dL at the final PK visit were 88%, 4% & 0%, respectively
- In exploratory EPs, improvements in QoL, energy/fatigue, erectile function, intercourse satisfaction, and positive mood were observed
Related Post: Insights+: The US FDA New Drug Approvals in June 2022


